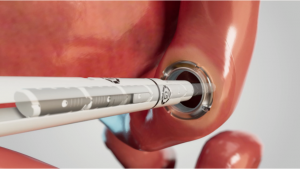
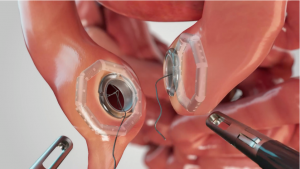
WESTWOOD, MA, UNITED STATES, August 4, 2025 /EINPresswire.com/ — GI Windows Surgical, a leader in next-generation medical devices, today announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Otoloc Access System which controls and captures the enterotomy between two Self-forming Magnets, allowing for an immediate lumen (1). GI Windows Surgical is designing a revolutionary anastomotic technology that enables less invasive surgery for patients, marking the first significant breakthrough in the field in over 40 years.
This FDA clearance marks a significant milestone in surgical innovation and introduces a novel technology with clinical promise, to disrupt the standard of care of staplers and sutures used in minimally invasive surgery and in endoluminal surgery.
“The Otoloc System is designed to capture and control an immediate lumen between two segments of the gastrointestinal tract using GI Windows’ patented magnetic compression technology” said Dr Erik Wilson, Chief Medical Officer at GI Windows Surgical. “This innovative approach reduces procedural complexity and has the potential to offer patients a faster and safer recovery pathway in both minimally invasive surgery and in the emerging endoluminal surgery category”.
Brian Tinkham, Chief Executive Officer of GI Windows Surgical, emphasized the significance of this regulatory milestone: “We are excited for the next phase of our journey. The FDA’s clearance of our system underscores our commitment to delivering truly transformative solutions. We will continue to create the clinical evidence required to support broader adoption”
About GI Windows Surgical:
GI Windows Surgical is the global leader in a new treatment category creating magnetic anastomoses with less invasive delivery of self-forming magnets. A Massachusetts based medical device company dedicated to developing the first fundamental breakthrough in anastomoses technology in both delivery and tissue fusion, located in Westwood, MA.
(1) U.S. Food and Drug Administration. 510(k) K250541 Clearance for Flexagon Plus OTOLoc Magnetic Compression Anastomosis System . August 1, 2025.
Contact
GI Windows Surgical
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content “as is” without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()